Standard Enthalpy Of Formation Of White Phosphorus . 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. the two values of p −⊖− p − ⊖ − are within 1% of each other, since 1 atm = 101.325 kpa.︎. There are some exception, such as phosphorus, for. it is correct that white phosphorus is usually used as the standard state for tabulating thermodynamic data, in particular. under standard conditions it is more stable than white phosphorus, but less stable than the thermodynamically stable black phosphorus. however, a comparison of the reported enthalpies of formation and gibbs energies of formation for key phosphate.
from www.numerade.com
the two values of p −⊖− p − ⊖ − are within 1% of each other, since 1 atm = 101.325 kpa.︎. it is correct that white phosphorus is usually used as the standard state for tabulating thermodynamic data, in particular. There are some exception, such as phosphorus, for. under standard conditions it is more stable than white phosphorus, but less stable than the thermodynamically stable black phosphorus. however, a comparison of the reported enthalpies of formation and gibbs energies of formation for key phosphate. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and.
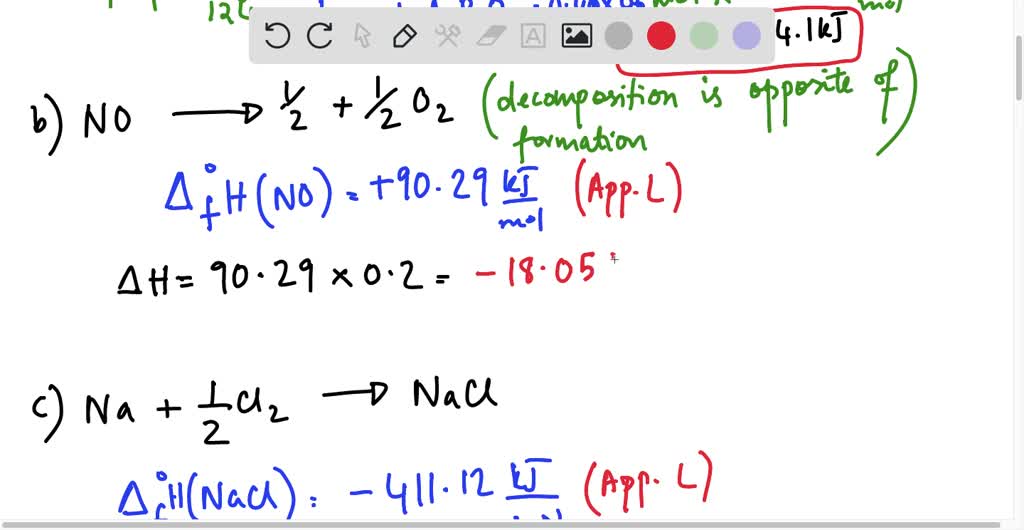
SOLVEDUse standard enthalpies of formation in Appendix L to calculate
Standard Enthalpy Of Formation Of White Phosphorus the two values of p −⊖− p − ⊖ − are within 1% of each other, since 1 atm = 101.325 kpa.︎. There are some exception, such as phosphorus, for. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. under standard conditions it is more stable than white phosphorus, but less stable than the thermodynamically stable black phosphorus. it is correct that white phosphorus is usually used as the standard state for tabulating thermodynamic data, in particular. the two values of p −⊖− p − ⊖ − are within 1% of each other, since 1 atm = 101.325 kpa.︎. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. however, a comparison of the reported enthalpies of formation and gibbs energies of formation for key phosphate.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Of White Phosphorus There are some exception, such as phosphorus, for. the two values of p −⊖− p − ⊖ − are within 1% of each other, since 1 atm = 101.325 kpa.︎. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. the standard enthalpy. Standard Enthalpy Of Formation Of White Phosphorus.
From www.youtube.com
standard_enthalpies_of_formation YouTube Standard Enthalpy Of Formation Of White Phosphorus the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. it is correct that white phosphorus is usually used as the standard state for tabulating thermodynamic data, in particular. however, a comparison of the reported enthalpies of formation and gibbs energies of formation. Standard Enthalpy Of Formation Of White Phosphorus.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Of White Phosphorus the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. however, a comparison of the reported enthalpies of formation and gibbs energies of formation for key phosphate. it is correct that white phosphorus is usually used as the standard state for tabulating. Standard Enthalpy Of Formation Of White Phosphorus.
From www.numerade.com
SOLVEDUse standard enthalpies of formation in Appendix L to calculate Standard Enthalpy Of Formation Of White Phosphorus the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. however, a comparison of the reported enthalpies of formation and gibbs energies of formation for key phosphate. under standard conditions it is more stable than white phosphorus, but less stable than the thermodynamically. Standard Enthalpy Of Formation Of White Phosphorus.
From www.numerade.com
SOLVED Define standard enthalpy change of combustion When red Standard Enthalpy Of Formation Of White Phosphorus the two values of p −⊖− p − ⊖ − are within 1% of each other, since 1 atm = 101.325 kpa.︎. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. the standard enthalpy of formation is defined as the change in enthalpy when one. Standard Enthalpy Of Formation Of White Phosphorus.
From askfilo.com
The standard enthalpies of formation of C2 H5 OH(1),CO2 ( g) and H2 O(1) Standard Enthalpy Of Formation Of White Phosphorus the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. the two values of p −⊖− p − ⊖ − are within 1% of each other, since 1 atm = 101.325 kpa.︎. it is correct that white phosphorus is usually used as the. Standard Enthalpy Of Formation Of White Phosphorus.
From exokycsnc.blob.core.windows.net
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog Standard Enthalpy Of Formation Of White Phosphorus 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. however, a comparison of the reported enthalpies of formation and gibbs. Standard Enthalpy Of Formation Of White Phosphorus.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Of White Phosphorus under standard conditions it is more stable than white phosphorus, but less stable than the thermodynamically stable black phosphorus. it is correct that white phosphorus is usually used as the standard state for tabulating thermodynamic data, in particular. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in. Standard Enthalpy Of Formation Of White Phosphorus.
From exoyndeil.blob.core.windows.net
Standard Enthalpy Of Formation In Elements at Michael Zapien blog Standard Enthalpy Of Formation Of White Phosphorus it is correct that white phosphorus is usually used as the standard state for tabulating thermodynamic data, in particular. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. the two values of p −⊖− p − ⊖ − are within 1%. Standard Enthalpy Of Formation Of White Phosphorus.
From narodnatribuna.info
Enthalpies Of Formation And Melting Points Of Iiiv Standard Enthalpy Of Formation Of White Phosphorus it is correct that white phosphorus is usually used as the standard state for tabulating thermodynamic data, in particular. There are some exception, such as phosphorus, for. however, a comparison of the reported enthalpies of formation and gibbs energies of formation for key phosphate. the standard enthalpy of formation is defined as the change in enthalpy when. Standard Enthalpy Of Formation Of White Phosphorus.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Of White Phosphorus however, a comparison of the reported enthalpies of formation and gibbs energies of formation for key phosphate. the two values of p −⊖− p − ⊖ − are within 1% of each other, since 1 atm = 101.325 kpa.︎. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a. Standard Enthalpy Of Formation Of White Phosphorus.
From docslib.org
Enthalpy of Formation of Phosphorus Pentachloride; Derivation of the Standard Enthalpy Of Formation Of White Phosphorus There are some exception, such as phosphorus, for. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. it is correct that. Standard Enthalpy Of Formation Of White Phosphorus.
From exokycsnc.blob.core.windows.net
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog Standard Enthalpy Of Formation Of White Phosphorus it is correct that white phosphorus is usually used as the standard state for tabulating thermodynamic data, in particular. There are some exception, such as phosphorus, for. however, a comparison of the reported enthalpies of formation and gibbs energies of formation for key phosphate. the standard enthalpy of formation is given the symbol δfho δ f h. Standard Enthalpy Of Formation Of White Phosphorus.
From exoxhphey.blob.core.windows.net
Standard Enthalpy Of Formation Calculator at Susan blog Standard Enthalpy Of Formation Of White Phosphorus the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. however, a comparison of the reported enthalpies of formation and gibbs. Standard Enthalpy Of Formation Of White Phosphorus.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Enthalpy Of Formation Of White Phosphorus under standard conditions it is more stable than white phosphorus, but less stable than the thermodynamically stable black phosphorus. however, a comparison of the reported enthalpies of formation and gibbs energies of formation for key phosphate. it is correct that white phosphorus is usually used as the standard state for tabulating thermodynamic data, in particular. There are. Standard Enthalpy Of Formation Of White Phosphorus.
From www.chemistryspace.com
Standard Enthalpy of Formation Standard Enthalpy Of Formation Of White Phosphorus it is correct that white phosphorus is usually used as the standard state for tabulating thermodynamic data, in particular. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. however, a comparison of the reported enthalpies of formation and gibbs energies of. Standard Enthalpy Of Formation Of White Phosphorus.
From mungfali.com
Enthalpies Of Formation Chart Standard Enthalpy Of Formation Of White Phosphorus the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. however, a comparison of the reported enthalpies of formation and gibbs energies of formation for key phosphate. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. Standard Enthalpy Of Formation Of White Phosphorus.
From www.numerade.com
SOLVEDUse standard enthalpies of formation in Appendix L to calculate Standard Enthalpy Of Formation Of White Phosphorus however, a comparison of the reported enthalpies of formation and gibbs energies of formation for key phosphate. it is correct that white phosphorus is usually used as the standard state for tabulating thermodynamic data, in particular. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that. Standard Enthalpy Of Formation Of White Phosphorus.